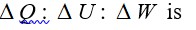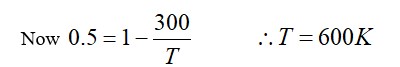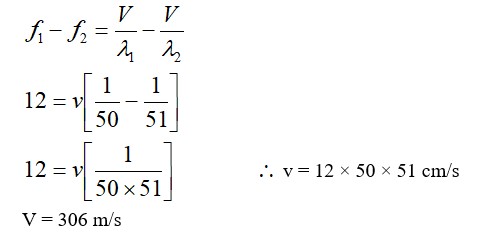If one mole of the polyatomic gas is having two vibrational modes and β is the ratio of molar specific heats for polyatomic gas (β = C_p / C_v), then the value of β is:
If one mole of the polyatomic gas is having two vibrational modes and β is the ratio of molar specific heats for polyatomic gas (β = C_p / C_v), then the value of β is:
In an LCR series AC circuit, the phase difference φ between current and voltage is: φ = tan? ¹ (X? - X? ) / R)
If each vibrational mode contributes two degrees of freedom and the total degrees of freedom f = 3 + 3 + 4 = 10, then:
β = 1 + 2/f = 1 + 2/10 = 1.2
Similar Questions for you
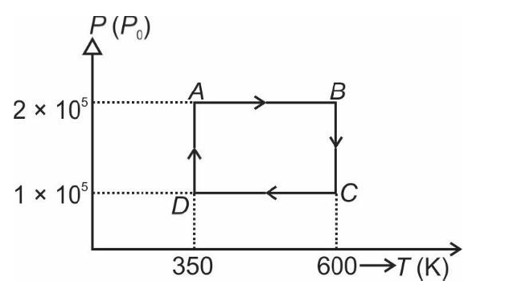
From A to B the process is isobaric
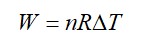
= W = 2 × R (600 - 350)
= 500 R
Heat is path dependent so path function but internal energy does not depend on path chosen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering