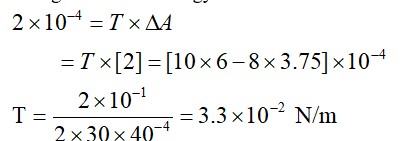Imagine removing one electron from He4 and He3. Their energy levels, as worked out on the basis of Bohr model will be very close. Explain why.
Imagine removing one electron from He4 and He3. Their energy levels, as worked out on the basis of Bohr model will be very close. Explain why.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- On removing one electron from He4 and He3, the energy levels, as worked out on the basis of Bohr model will be very close as both the nuclei are very heavy as compared to electron mass.Also after removing one electron
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
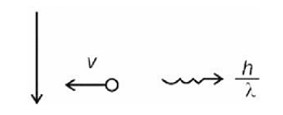
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering