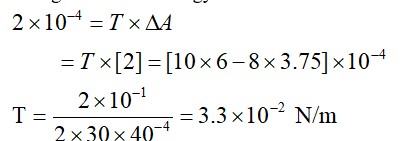In the Auger process, an atom maizes a transition to a lower state without emitting a photon. The excess energy is transferred to an outer electron which may be ejected by the atom (This is called an Auger electron). Assuming the nucleus to be massive, calculate the kinetic energy of an n = 4 Auger electron emitted by Chromium by absorbing the energy from a n – 2 to n = 1 transition.
In the Auger process, an atom maizes a transition to a lower state without emitting a photon. The excess energy is transferred to an outer electron which may be ejected by the atom (This is called an Auger electron). Assuming the nucleus to be massive, calculate the kinetic energy of an n = 4 Auger electron emitted by Chromium by absorbing the energy from a n – 2 to n = 1 transition.
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- the energy of nth state En=-Z2R where R is constant and Z=24
The energy release ina transition from 2 to 1
The energy required to eject a n=4 electron is E4= Z2R1/16
So kinetic energy of auger electron is, KE= Z2R (3/4
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering