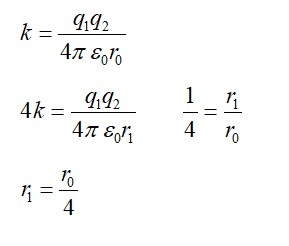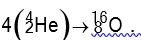In the hydrogen spectrum, be the wavelength of first transition line of Lyman series. The wavelength difference will be between the wavelength of 3rd transition line of Paschen series and that 2nd transition line B Balmer series where a = __________.
In the hydrogen spectrum, be the wavelength of first transition line of Lyman series. The wavelength difference will be between the wavelength of 3rd transition line of Paschen series and that 2nd transition line B Balmer series where a = __________.
-
1 Answer
-
For first line of Lyman
3rd line (Paschen)
2nd line (B almer)
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers