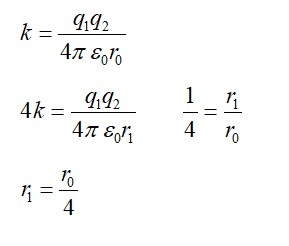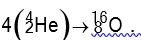Nuclei with magic no. of proton Z=2,8,20,28,50,52 and magic no. of neutrons N=2,8,20,28,50,82 and 126 are found to be stable. (i) Verify this by calculating the proton separation energy Sp for. 120Sn ( Z = 50 ) and 121Sb( Z = 51 ). The proton separation energy for a nuclide is the minimum energy required to separated the least tightly bound proton form a nucleus of that nuclide. It is given by Sp = ( Mz-1 , N + MH−Mz ,N )c2 𝑆𝑝 = ( 𝑀𝑧 - 1 , 𝑁 + 𝑀H – 𝑀Z , 𝑁 ) 𝑐 2 . given 119𝑆𝑛 = 118.9058 𝑢 , . 120𝑆𝑛 = 199.902199 , . 121𝑆𝑏 = 120.903824 𝑢 ,1𝐻 = 1.0078252 𝑢 (ii) what does the existence of magic number indicate?
Nuclei with magic no. of proton Z=2,8,20,28,50,52 and magic no. of neutrons N=2,8,20,28,50,82 and 126 are found to be stable. (i) Verify this by calculating the proton separation energy Sp for. 120Sn ( Z = 50 ) and 121Sb( Z = 51 ). The proton separation energy for a nuclide is the minimum energy required to separated the least tightly bound proton form a nucleus of that nuclide. It is given by Sp = ( Mz-1 , N + MH−Mz ,N )c2 𝑆𝑝 = ( 𝑀𝑧 - 1 , 𝑁 + 𝑀H – 𝑀Z , 𝑁 ) 𝑐 2 . given 119𝑆𝑛 = 118.9058 𝑢 , . 120𝑆𝑛 = 199.902199 , . 121𝑆𝑏 = 120.903824 𝑢 ,1𝐻 = 1.0078252 𝑢 (ii) what does the existence of magic number indicate?
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- B.E = (Mp+MH-MN)c2
B.E= (118.9058+1.0078252-119.902199)c2
B.E=0.0114362 c2
B.E= (Mp+MH-MN)c2
B.E= (119.902199+1.0078252-120.902822)c2
B.E= 0.0059912c2
(ii) the existence of magic numbers indicates that the shell structure o
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering