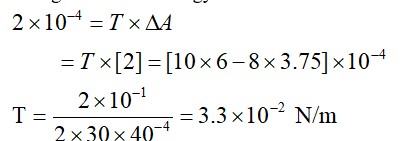Show that the first few frequencies of light that is emitted when electrons fall to nth level from levels higher than n, are approximate harmonics (i.e., in the ratio 1:2:3 …) when n >> 1.
Show that the first few frequencies of light that is emitted when electrons fall to nth level from levels higher than n, are approximate harmonics (i.e., in the ratio 1:2:3 …) when n >> 1.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- The frequency of any line in a series in the spectrum of hydrogen like atoms corresponding to the transition of electrons from (n+ p) level to nth level can be expressed as a difference of two terms;
From the formula we
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering