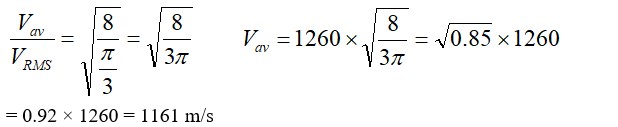The container shown in Fig. has two chambers, separated by a partition, of volumes V1 = 2.0 litre and V2= 3.0 litre. The chambers contain µ1 = 4.0and µ2 =5.2 moles of a gas at pressures p1= 1.00 atm and p2 = 2.00 atm. Calculate the pressure after the partition is removed and the mixture attains equilibrium.
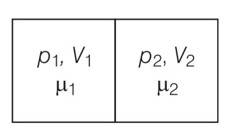
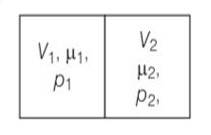
The container shown in Fig. has two chambers, separated by a partition, of volumes V1 = 2.0 litre and V2= 3.0 litre. The chambers contain µ1 = 4.0and µ2 =5.2 moles of a gas at pressures p1= 1.00 atm and p2 = 2.00 atm. Calculate the pressure after the partition is removed and the mixture attains equilibrium.


This is a short answer type question as classified in NCERT Exemplar
V1=2L ,V2=3L
µ1 = 4.0and µ2 =5.2
p1= 1.00 atm and p2 = 2.00 atm
p1V1= µ1RT1
p2V2= µ2RT2
when the partition is removed the gases get mixed without any loss of energy . the mixture now attains a common equilibrium pressure and total volume
Similar Questions for you
PV = nRT
->Pµn
->Ratio=
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

physics ncert solutions class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering