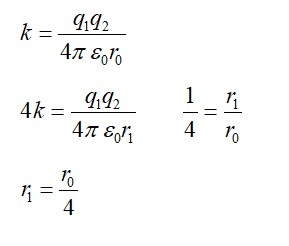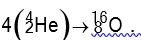The half-life of Au¹⁹⁸ is 2.7 days. The activity of 1.50 mg of Au¹⁹⁸ if its atomic weight is 198 g mol⁻¹ is, (Nₐ = 6×10²³ / mol)
The half-life of Au¹⁹⁸ is 2.7 days. The activity of 1.50 mg of Au¹⁹⁸ if its atomic weight is 198 g mol⁻¹ is, (Nₐ = 6×10²³ / mol)
Option 1 -
240 Ci
Option 2 -
252 Ci
Option 3 -
535 Ci
Option 4 -
357 Ci
-
1 Answer
-
Correct Option - 4
Detailed Solution:A = Activity = λN = (ln (2)/t? /? ) N
N = (1.5×10? ³ / 198) × 6×10²³
A = (0.693 / (2.7×24×3600) × (1.5×10? ³ / 198) × 6×10²³ disintegration/s
A ≈ 1.32 × 10¹³ Bq = (1.32×10¹³ / 3.7×10¹? ) Ci ≈ 357 Ci
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers