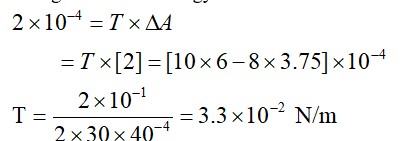What is the difference between atomic absorption and emission spectra?
What is the difference between atomic absorption and emission spectra?
When white light passes through a cool gas, atoms absorb specific wavelengths. This produces a continuous background with dark lines at those absorbed wavelengths. This is atomic absorption spectra. But when atoms are excited, they release photons at specific wavelengths, producing a dark background
Similar Questions for you
Every element has a unique set of spectral lines as its electrons occupy specific energy levels. What scientists know for certain is that these unique patterns act like fingerprints. It helps in identifying elements in stars, flames, or unknown samples. For instance, Helium was discovered in the Sun
We see a discrete emission spectrum when electrons inside excited atoms or ions in gases fall back from higher energy levels to lower ones. Every transition releases a photon of a specific wavelength. Usually the spectrum appears as sharp, bright lines. Dark gaps separate them. These lines are uniqu
Kindly go through the solution
Change in surface energy = work done
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Atoms 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering