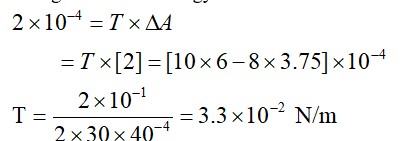What series of lines are observed in the hydrogen spectrum?
What series of lines are observed in the hydrogen spectrum?
The hydrogen emission spectrum contains several spectral series, each named after its discoverer.
- Lyman series (n' = 1): To ground state, visible only in ultraviolet region
- Balmer series (n' = 2): Transitions to second level, appearing in visible region
- Paschen series (n' = 3): Moved to third level, vi
Similar Questions for you
Hydrogen shows many spectral lines because of the following reasons.
Its electron can occupy many levels (n = 1, 2, 3.).
Each line in the hydrogen spectrum actually represents the transition from higher to lower energy levels.
These lines are grouped by the final energy level (Lyman
Hydrogen produces a line spectrum because electrons exist only in discrete, quantised energy levels. When electrons jump between these fixed energy states, they emit photons with specific energies (E = h? ). This creates distinct spectral lines instead of continuous wavelengths. Bohr's model explain
Kindly go through the solution
Change in surface energy = work done
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Atoms 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering