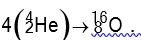When a nucleus in an atom undergoes a radioactive decay, the electronic energy levels of the atom
(a) Do not change for any type of radioactivity
(b) Change for α and β-radioactivity but not for γ-radioactivity
(c) Change for α -radioactivity but not for others
(d) Change for β-radioactivity but not for others
When a nucleus in an atom undergoes a radioactive decay, the electronic energy levels of the atom
(a) Do not change for any type of radioactivity
(b) Change for α and β-radioactivity but not for γ-radioactivity
(c) Change for α -radioactivity but not for others
(d) Change for β-radioactivity but not for others
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (b)
Explanation – because alpha and beta particle have some charge and mass so their energy level will change but in case of gamma particle it does not contain any charge.
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering