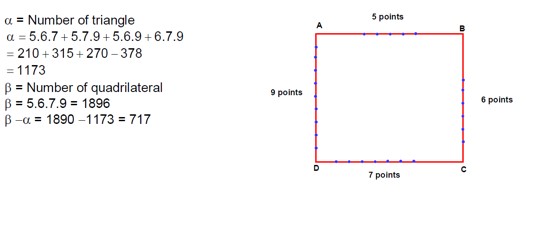
Ask & Answer: India's Largest Education Community
All Questions
New Question
5 months agoContributor-Level 9
The complex CoCl? ·4NH? is represented as [CoCl? (NH? )? ]Cl.
Here, 4 NH? are neutral monodentate ligands. So, 2 equivalents of ethylene diamine replace 4NH? since ethylene diamine is a didentate ligand.
New Question
5 months agoContributor-Level 9
ΔG° = -9.478 kJ/mol
Using ΔG° = -2.303 RT log K_p
-9.478 × 10³ = -2.303 × 8.314 × 495 log K_p
1 = log K_p ⇒ K_p = 10
Here, for the given reaction A (g)? B (g), K_p = K_c
Initial A = 22 mmol
At equilibrium A = 22 - x mmol; B = x mmol
K_c = [B] / [A] = (x/V) / (22-x)/V) = x / (22-x) = 10
x = 10 (22-x) ⇒ x = 220 - 10x ⇒ 11x = 220 ⇒ x = 20
So, mmol of B at equilibrium are 20.
New Question
5 months agoContributor-Level 9
m = 10 molal
K_b = 0.5 K kg mol? ¹
Using: ΔT_b = I K_b m
and α = (i - 1) / (n - 1)
n for AB? is 3; α = 0.1
0.1 = (i - 1) / (3 - 1) ⇒ I = 1.2
ΔT_b = 1.2 × 0.5 × 10 = 6 °C
So, boiling point of solution = 100 + 6 = 106 °C
New Question
5 months agoContributor-Level 7
The CEETA PG 2026 admit card will be released on the official website after the registration process. The expected CEETA PG 2026 hall ticket release date is the first week of March 2026. Candidates are advised to check the website for the latest updates and notifications.
New Question
5 months agoContributor-Level 9
A 6.5 molal solution means 6.5 moles of KOH is in 1 kg (1000 g) of solvent (H? O).
Moles of solute, n_B = 6.5
Mass of solute, W_B = 6.5 × 56 = 364 g
Mass of solvent, W_A = 1000 g
Mass of solution = 1364 g
Volume of solution = 1364 / 1.89 mL
Now, molarity = [6.5 / (1364 / 1.89)] × 1000 M = 9 M
New Question
5 months agoContributor-Level 9
Moles of carbon in organic compound = Moles of carbon in CO?
n_c = 420 / 44 moles
Mass of carbon in organic compound = (420 / 44) × 12 = 114.54 g
Moles of hydrogen in compound = 2 × moles of H? O
n_H = 2 × (210 / 18) moles
Mass of hydrogen = 2 × (210 / 18) g = 23.33 g
% of H = (23.33 / 750) × 100 = 3.11%
The nearest integer is 3.
New Question
5 months agoBeginner-Level 1
Visit the SendWishOnline website.
Start by going to the homepage and selecting the option to create a new group card.Choose a Spanish birthday template.
Browse through the available designs and pick a Spanish birthday card that suits your celebration theme.Customize your card.
Add a title, recipient’s name, and a personalized message in Spanish. You can also change the font, colors, and add emojis or GIFs to make it more festive.Invite others to sign.
Once your card is ready, share the unique card link with your friends, family, or coworkers so they can add their own messages, photos, or wishes in Spanish.Send or schedule the card.
Af
New Question
5 months agoContributor-Level 9
Solubility product of A? X = 4S? ³
Where S? is the solubility of salt A? X.
Solubility product of MX = S? ²
Where S? is the solubility of MX.
Given 4S? ³ = 4 × 10? ¹² ⇒ S? = 10? M
Given S? ² = 4 × 10? ¹² ⇒ S? = 2 × 10? M
So, S? / S? = 10? / (2 × 10? ) = 50
New Question
5 months agoContributor-Level 9
The balanced equation is:
2MnO? + 5C? O? ²? + 16H? → 2Mn²? + 10CO? + 8H? O
So, the value of c (coefficient for H? ) = 16.
New Question
5 months agoContributor-Level 10
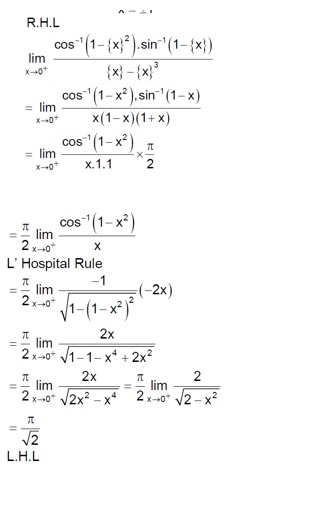
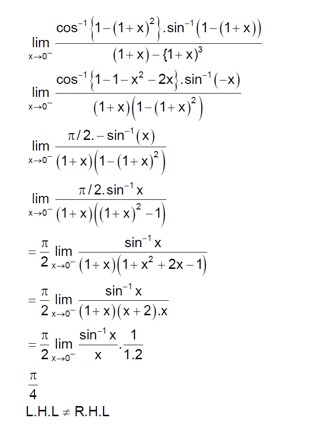
Solve sin? ¹ (3x/5) + sin? ¹ (4x/5) = sin? ¹x.
Using the formula sin? ¹a + sin? ¹b = sin? ¹ (a√ (1-b²) + b√ (1-a²):
sin? ¹ ( (3x/5)√ (1 - (4x/5)²) + (4x/5)√ (1 - (3x/5)²) ) = sin? ¹x
(3x/5) * √ (1 - 16x²/25) + (4x/5) * √ (1 - 9x²/25) = x
x * [ (3/5) * √ (25-16x²)/5 + (4/5) * √ (25-9x²)/5 - 1 ] = 0
So, x = 0 is one solution.
For the other part:
3√ (25-16x²) + 4√ (25-9x²) = 25
Let's check integer solutions. If x = 1:
3√ (9) + 4√ (16) = 33 + 44 = 9 + 16 = 25. So x = 1 is a solution.
If x
New Question
5 months agoContributor-Level 9
Edge length in bcc, a? = 27 Å
Let, Edge length in fcc be a? Å
Now, the same element crystallises in bcc as well as fcc.
For bcc: 4r = √3 a? ⇒ r = (√3 / 4) a?
For fcc: 4r = √2 a? ⇒ r = a? / (2√2)
So, (√3 / 4) a? = a? / (2√2)
(√3 / 4) × 27 = a? / (2√2)
a? = 33.13 Å
The nearest integer is 33.
New Question
5 months agoContributor-Level 9
There are around 6,000 MA colleges in India, and every college offers different specializations in the MA. Also, every college has a different college rating, placement record, faculty rating, etc., based on that, the course fee structure of these colleges is decided. Hence, the fee structure is vast.
It can start from thousands and go up to lakhs. In the case of MA course fees in India, the average starts from INR 1,000 and goes up to INR 14.7 Lakh.
know more about -
New Question
5 months agoGuide-Level 15
IIM Amritsar offers a five-year Integrated programme in Management or IPM leading to MBA. Students in the course get an option to exit the course in three-years with a Bachelor of Science in Quantitative Finance and Economics degree. Students are shortlisted for IPM based on their IPMAT scores. Selected candidates have to further pass a personal interview round for admission.
New Question
5 months agoContributor-Level 9
H? O? can act as both an oxidizing & reducing agent in both acidic & basic medium. In the hydrogen economy, energy is stored & transmitted in the form of dihydrogen.
New Question
5 months agoContributor-Level 9
Roasting is a process in which sulphur is removed as SO? gas from sulphide ores on heating in excess of oxygen.
New Question
5 months agoContributor-Level 9
Bismuth (Bi) is a metal in group 15. Its hydride is BiH? , which is the strongest reducing agent due to its the lowest thermal stability.
New Question
5 months agoContributor-Level 9
Hydrated halides of group 2 from Ca onwards undergo dehydration on heating. But the hydrated halide of Mg, i.e., MgCl? ·8H? O, does not undergo dehydration on heating but undergoes hydrolysis.
BeO is amphoteric, while other oxides of group 2 are basic in nature.
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts