Ask & Answer: India's Largest Education Community
All Questions
New Question
4 months agoContributor-Level 10
Yes, JIIT, Online admits students based on Class 12 scores. The institute admits students based on merit. Students can check the table below to know the detailed course eligibility:
| Course Name | Eligibility |
|---|---|
| BBA | Class 12 |
| MBA | Graduation |
New Question
4 months agoContributor-Level 10
To secure admission at JIIT, Online, candidates must complete graduation as the basic eligibility criteria. Aspirants can check the points mentioned below to know more:
- Aspirants can visit the official website
- Apply online for admission
- Students are selected based on merit
New Question
4 months agoContributor-Level 10
IMT CDL provides PG and certificate programmes to students. At the postgraduate level, IMT CDL offers a two-year PGDM, a 15-month Executive PGDM, and a 13-month PGCM course to students. Some of the certificate programmes available at the IMT CDL include Proficiency Certificate Programme in Financial Management, Proficiency Certificate Programme in Human Resource Management, and others. Below mentioned are some of the IMT CDL courses and their respective eligibility and IMT CDL fee:
New Question
4 months agoContributor-Level 10
Each year, Duke receives over 50,000 applications, of which the university admits a mere 2,800-3,000 students. The UG admission trends at Duke over the years can be seen in the table given below:
Class | Number of Applications Received | Number of Students Accepted | Duke University Acceptance Rate |
|---|---|---|---|
2029 | 59,850 | 2,802 | 4.8% |
2028 | 54,194 | 2,925 | 5.4% |
2027 | 49,476 | 3,115 | 6.3% |
2026 | 50,002 | 3,085 | 6.17% |
2025 | 49,517 | 2,854 | 5.7% |
New Question
4 months agoNew Question
4 months agoNew Question
4 months agoContributor-Level 9
If size of object is very small as compare to wave length of EM wave in free space then, scattering will happen.
New Question
4 months agoContributor-Level 8
No, right now admissions are not open in the Siddharth College of Law. But I have an existing opportunity available for you right now only if you are a female student. You can apply for admission at Shanti Devi arya mahila college, Dinanagar, District Gurdaspur. Right now is the examination time but still you can get admission here. Consult me for more details.
New Question
4 months agoContributor-Level 9
In an oxidation-reduction reaction or redox reaction, the process of oxidation (addition of electrons) and reduction (removal of electrons) happens at the same time.
New Question
4 months agoContributor-Level 9
Redox reaction does not have a fixed formula but is an equation that forms when oxidation and reduction occur simultaneously. For example, 2Fe + 3Cl? 2FeCl? (Fe loses electrons and Cl gains).
New Question
4 months agoContributor-Level 9
Redox reactions are of four kinds: Decomposition, Combination, Displacement, and disproportionation reactions.
New Question
4 months agoContributor-Level 10
Due to H- bond in water, it has high melting point and melting point of other hydrides of the group are depending upon the molecular weight.
New Question
4 months agoContributor-Level 10
Due to high crystallity Be has the highest M.P.
Be = 1560 K
Mg = 925 K
Ca = 1120 K
Sr = 1062 K
New Question
4 months agoContributor-Level 10
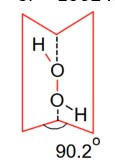
During the electrolysis of dilute H2SO4
In the solid form of dihedral angle is equal to 90.2°.
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts