Ask & Answer: India's Largest Education Community
All Questions
New Question
4 months agoBeginner-Level 4
B.Tech in CSE at Chandigarh University is well-structured to provide students with both fundamental concepts and cutting-edge technology experience. The programme includes hands-on training in AI, data analytics, and full-stack development, often supported by IBM's industry network. Students work on live projects using real datasets, which enhances problem-solving skills and practical knowledge. CU's placement cell connects students with a variety of recruiters, from multinational corporations to growing startups. Companies like Microsoft, Intel, Juspay, Axtria, and Sabre Technologies are regular visitors, looking for talent in softwar
New Question
4 months agoBeginner-Level 3
Chandigarh University is a strong choice for software engineering due to its industry-oriented curriculum and hands-on learning approach. Students are trained in programming, software development, and system analysis using labs, live projects, and real-world tools. The university also provides specialised programs like CSE with IBM, which adds advanced training in AI, ML, and data analytics. Experienced faculty guide students through both theoretical and practical knowledge, supported by modern infrastructure including labs, libraries, and CU's LMS, where all content is digitally accessible. Regular workshops, seminars, and industry co
New Question
4 months agoContributor-Level 6
Chandigarh University excels in both Chemical and Computer Engineering programs. Chemical Engineering provides solid foundations in process design, environmental engineering, and industrial applications. The programme includes practical labs and electives in petroleum and environmental engineering. Computer Engineering, meanwhile, focuses on software, systems, and data analytics, with options like the IBM-specialized CSE programme for students wanting deeper expertise in AI and analytics. With strong faculty, modern labs, and NIRF 31 ranking, CU ensures students in both fields are industry-ready. Students interested in tech, software,
New Question
4 months agoBeginner-Level 4
Yes, Chandigarh University is highly suitable for Computer Science studies. The university offers multiple specialised programs in CSE, BCA, and related fields, integrating modern technologies like AI, AR/VR, and data science. Labs are fully equipped for hands-on learning, and students can work on live projects and industry-oriented assignments. CU's programs are designed to balance theoretical knowledge with practical experience, allowing students to learn programming, software development, and data analytics while gaining exposure to tools like SQL, Python, and Tableau. The university also encourages participation in workshops, hacka
New Question
4 months agoBeginner-Level 4
If you're looking at CSE at Chandigarh University, both programs are strong. The regular CSE course offers a comprehensive understanding of computer Science fundamentals, programming, and system design. In contrast, CSE with IBM is a globally recognised programme co-designed with IBM, featuring hands-on labs, experiential projects, and mentorship from IBM professionals. Students also get access to IBM's Learning Management System and practical exposure to industry tools and data analytics platforms. The IBM programme is ideal for those seeking specialised skills in AI, machine learning, and big data, whereas the standard CSE allows fle
New Question
4 months agoContributor-Level 10
Primary structure of protein in unaffected by physical or chemical changes.
New Question
4 months agoContributor-Level 10
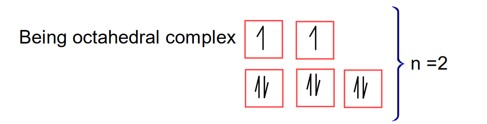
For precipitation of two moles of AgCl
Two Cl- will produce as a free anion
CoCl3.4NH3 -> complex will Cl (will not give 2Cl-)
complex will be H2 [PtCl6] will not any Cl-
will produce two Cl- ion.
precipitate formation
New Question
4 months agoContributor-Level 10
Most basic oxide V2O3
Here V has +3 O.S. Hence V+3 ->
two unpaired e- in d- subshell
New Question
4 months agoContributor-Level 10
Volume of H2 adsorbed =
Therefore volume of gas adsorbed per gram of the adsorbent =
New Question
4 months agoContributor-Level 9
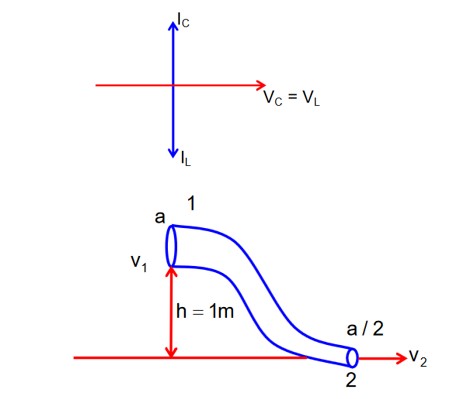
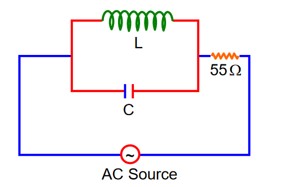
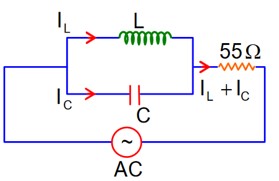
At Resonance
XL = XC
then lL = lC
Now phasor diagram
for L & C
So, Net current = zero
Therefore current through R circuit at resonance will be zero
New Question
4 months agoContributor-Level 10
Aniline show acid-base reaction with AlCl3
aniline is a Lewis base while AlCl3 acts as lewis acid.
New Question
4 months agoContributor-Level 10
Process is based upon simultaneous disintegration hence,
………….(i)
and ………….(ii)
from equation (i) and (ii)
Here; A0 = B0 and
Therefore
New Question
4 months agoNew Question
4 months agoContributor-Level 10
Here, total meq of acetic acid = 50 × 0.1 = 5
And total meq of NaOH = 25 × 0.1 = 2.5
After neutralization process
Meq of left acetic acid = 2.5
And meq of formed CH3COONa = 2.5
New Question
4 months agoContributor-Level 10
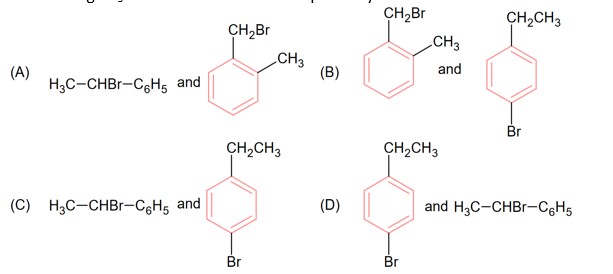
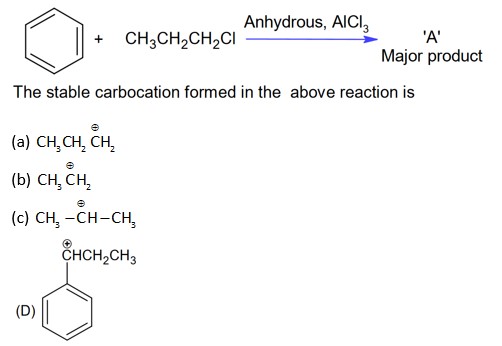
With AlCl3, alkyl halide will form cabocation which will show rearrangement.
New Question
4 months agoContributor-Level 10
Candidates looking for admission at Centre for Distance and Online Education, JIIT must complete at least a graduation degree for the MBA course. Similarly, for the BBA course, aspirants must complete Class 12.
New Question
4 months agoContributor-Level 9
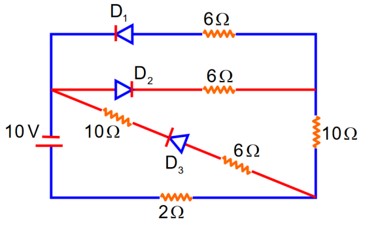
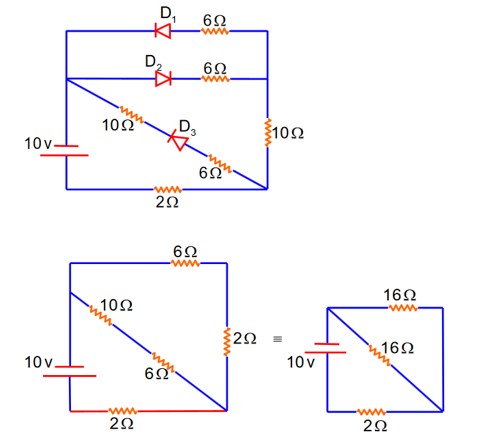
Forward biased offer zero Resistance
D2} Reversed biased offers Infinite Resistance
New Question
4 months agoContributor-Level 10
0.5 % KCl solution has molality (m) =
1 - a a a
And I =
1.976 = 1 + a
% = 97.6%
the nearest 98.
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts