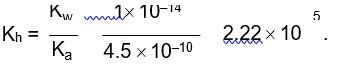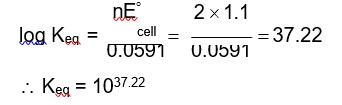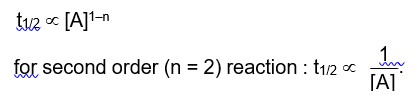Ask & Answer: India's Largest Education Community
All Questions
New Question
4 months agoNew Question
4 months agoNew Question
4 months agoNew Question
4 months agoContributor-Level 10
In physisorption multimolecular layers are formed on solid surface.
New Question
4 months agoContributor-Level 10
More the number of ions in solution, more will be the specific conductance
is more than that of Ionic conductivity of H? Na+
New Question
4 months agoNew Question
4 months agoContributor-Level 10
We consider the enthalpy change (delta H) more important than the internal energy (delta U) for practical reasons. When we go by the definitions alone, internal energy is the heat absorbed or released at a constant volume. This only happens in a sealed container. But in real life experiments held at laboratories, we have open vessels like test tubes, and there, only atmospheric pressure as a state variable remains constant, and not volume per se. This condition of constant pressure becomes more useful to calculate enthalpy in the chemical reaction.
New Question
4 months agoContributor-Level 10
We cannot universally say that negative enthalpy is the cause for spontaneity in chemical reactions. Though it is commonly seen in spontaneous reactions that heat releases or follows the exothermic definition, some spontaneous reactions can also absorb heat. They can be endothermic in nature. With that, we get a positive value for enthalpy change. So the better approach is to look into entropy and Gibbs Energy. These two quantities can define or tell us that a chemical process is spontaneous when the total entropy change is positive and when the Gibbs energy change is negative.
New Question
4 months agoContributor-Level 10
This can be a little confusing when we already know the equation from the First Law of Thermodynamics (delta U = q + W) has internal energy as a state function. Work (W) and heat (q) are dependent on the path entirely, but internal energy is only concerned with initial and final states. We can consider the example of work done for an extremely slow or quick process of a gas expanding. The work done for slow work will be different from that of a faster one, but their initial and final states won't have any effect.
New Question
4 months agoContributor-Level 10
Check the top industries for Boston Uni's MBA graduates mentioned below-
- Health & Life Sciences 22%
- Consulting Services 18%
- Financial Services 13%
- Technology 12%
- Energy 11%
- Retail 6%
- Non Profit 5%
- Manufacturing 5%
- Government 2%
- Real Estate 2%
- Transportation & Logistics 1%
- Education 1%
New Question
4 months agoContributor-Level 10
Yes, almost every programme at the institute is taught in English. Besides, every programme has electives in addition to its core curriculum. To learn more, check the courses and fees panel of Eindhoven University of Technology at Shiksha.
New Question
4 months agoNew Question
4 months agoContributor-Level 9
That's a great EMBA option. The Global MBA offered with Liverpool John Moores University through IMT combines practical business knowledge with international experience. The programme provides global case studies, leadership modules, as well as online learning flexibility for working professionals. IMT has strong relevance in Indian industry and LJMU has depth of academic knowledge and a UK degree. The program's design develops strategic thinking, a cross-cultural management skillset, and decision-making in real time. This option is a good fit if your intention is to grow your career, move into global roles, or consider entrepreneurshi
New Question
4 months agoNew Question
4 months agoContributor-Level 10
The average base salary of UCalgary MBA graduate is CAD 117,000 (INR 76 LPA) annually. According to the student reviews on Shiksha, University of Calgary graduates can earn from CAD 60,000 - 65,000 (INR 36.32 L - INR 39.35 L) per year. The university graduates work in top companies such as City of Calgary, Suncor, TC Energy, Cenovus Energy, SAIT, Mount Royal University, ATB Financial, and others.
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts