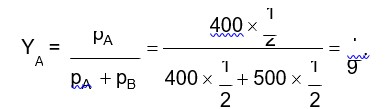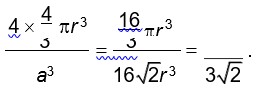Ask & Answer: India's Largest Education Community
All Questions
New Question
4 months agoContributor-Level 10
Yes international students can get a Post study work permit after studying at University of Calgary. They can apply for both work permits as well as co ops and internships.
International students who are enrolled in the International Professional Program at UCalgary Continuing Education and have finished their non degree credit courses can apply for a post-graduate work permit (PGWP).
New Question
4 months agoNew Question
4 months agoNew Question
4 months agoContributor-Level 10
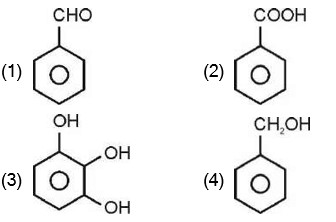
is optically active as it does not contain any element of symmetry.
New Question
4 months agoContributor-Level 10
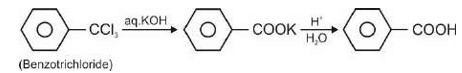
Correct order of increasing field strength of ligands is : I– < OH– < NCS–
New Question
4 months agoContributor-Level 10
EAN = Atomic No. – Oxidation state + 2 * CN
= 26 – 2 + 2 * 6
=36
New Question
4 months agoContributor-Level 10
E =
As temperature increases, strain increases
Elasticity decreases
New Question
4 months agoContributor-Level 10
Higher the value of (ixM), higher will be the boiling point of solution.
Solute | Glucose | NaCl | K2SO4 | K4 [Fe (N)6] |
van't Hoff factor (i) | 1 | 2 | 3 | 5 |
New Question
4 months agoContributor-Level 10
CsCl has BCC structure in which Cl– is present at corners of cube and Cs+ at body centre
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts