What are the differences between old and modern periodic table?
7 Views|Posted 8 months ago
Asked by Shiksha User
1 Answer
V
Answered by
8 months ago
The differences between old and modern periodic table are as follows:
| Mendeleev's Table (Old Periodic Table) | Modern Periodic Table (Long Form Table) |
| Arrangement of elements are done on atomics weights (mass) | Arrangement of elements are done on atomic number |
| Contains 66 elements | Contains 118 known elements |
| Noble Gases not mentioned in old periodic table because it was not discovered | Noble gases are included in Group 18 |
| Transistion elements were placed with other elements | Transition elements are placed in seperate block |
Similar Questions for you
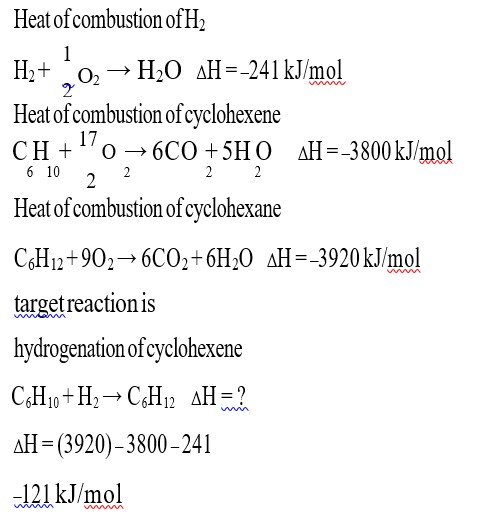
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.8L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering