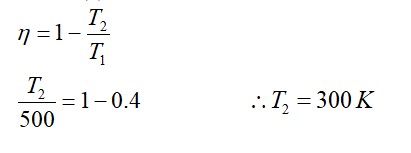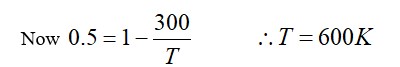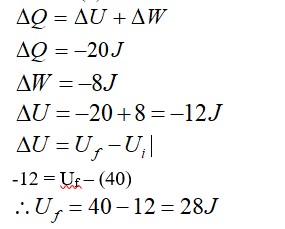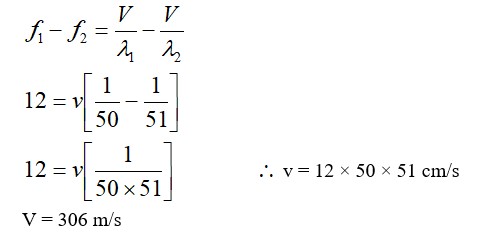What is entropy in Thermodynamics?
2 Views|Posted 10 months ago
Asked by Shiksha User
1 Answer
P
Answered by
10 months ago
A randomness or a measure of disorder in a system is called entropy. It is equal to the amount of unavailable energy for doing work in thermodynamics. According to the second law of Thermodynamics, entropy is an isolated system. It either remains constant or always increases. Entropy explains that p
Similar Questions for you
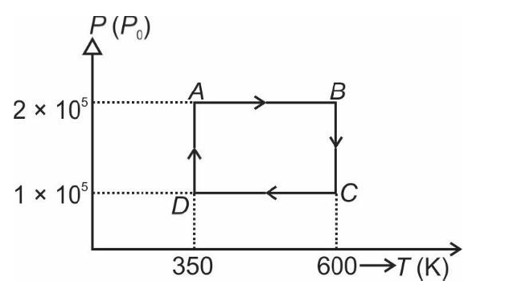
From A to B the process is isobaric
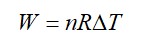
= W = 2 × R (600 - 350)
= 500 R
Heat is path dependent so path function but internal energy does not depend on path chosen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering