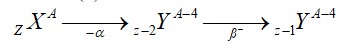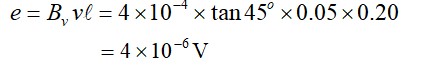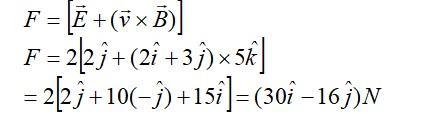What is the difference between aliphatic and aromatic amines?
-
1 Answer
-
Amines are prepared by replacing the hydrogen atom with alkyl or aryl groups from amonia (NH? ). Students can differntiate Aliphatic and aromatic amines differ based on the type of group attached to the nitrogen atom.
- Aliphatic amines have one or more alkyl groups (like methyl or ethyl) bonded to the nitrogen, such as ethylamine (CH? NH? ).
- Aromatic amines have an aryl group (like benzene) directly bonded to nitrogen, for example, aniline (C? H? NH? ).
Aliphatic amines are generally more basic than aromatic amines because, in aromatic amines, the lone pair on nitrogen is delocalized into the aromatic ring through resonanc
...more
Similar Questions for you
The direction of induced emf is to oppose changing flux.
From BF3 to BI3 Lewis acidic strength increases
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers