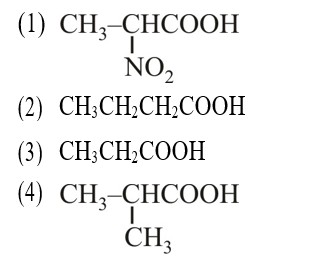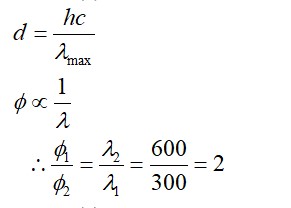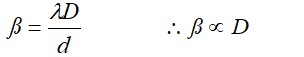What is the structure of the atom Class 11 Rutherford model?
-
1 Answer
-
The Rutherford model states that when a high energy Alpha particle is passed through a thin gold foil (100 nm thickness) by placing a fluorescent zinc sulphide screen around the foil, then most of the particles pass through the thin foil undeflected, while some of the particles are deflected by a small angle. However, very few particles bounced back, that is, were deflected by nearly 180°.
Similar Questions for you
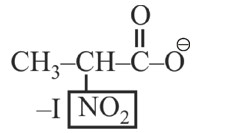
–I effect ∝ Acidic strength
+I effect ∝ Basic strength
* Most stable anion due to maximum –I effect.
* Most acidic
with increase in separation of screen from slits plane, fringe width increases.
Excessive nitrate in drinking water causes methemoglobinemia
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers