Many students wants to findout that even though phenol and alcohol both contains the hydroxy ion, then why formar is acidic than latter. Well, Here's the answer, Phenols are more acidic because the phenoxide ion formed after losing H? is stabilized by resonance. In contrast, alcohols do not have thi
Similar Questions for you
At room temperature Hg is liquid and it is purified by 'Distillation method'.
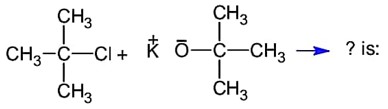
Base is tert-butoxide thus favours elimination with 3° alkyl halide and gives alkene
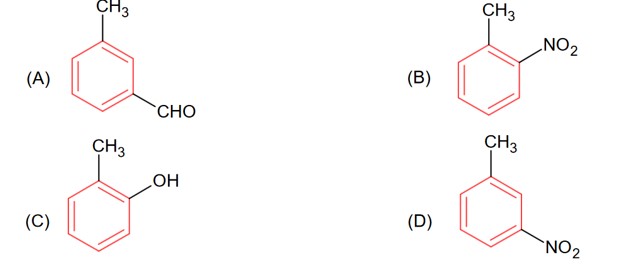
OH group is the more activating group and ortho-para director, hence ortho position attach will provide meta substituted product with respect to – CH3 in option (C).
Due to H- bond in water, it has high melting point and melting point of other hydrides of the group are depending upon the molecular weight.
Phenol is weakly acidic compound but it is more acidic than alcohol and water because its conjugate base is more stable. Hence statement -I is correct while statement -II is wrong.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Alcohols, Phenols and Ethers
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering