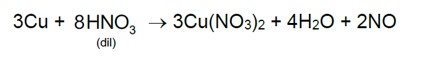7.29 Give the reason for bleaching action of Cl2.
7.29 Give the reason for bleaching action of Cl2.
-
1 Answer
-
7.29
Moist chlorine is a good reducing agent because it can accept electrons from other species as it is very electronegative.
(iii) The bleaching action of chlorine is due to oxidation by nascent oxygen produced. This nascent oxygen can be produced by
Chlorine dissolves in water in absence of sunlight and forms hydrochloric acid and hypochlorous acid (HOCl)
Since hypochlorous acid is not much stable it decomposes giving nascent oxygen. Cl2 + H2O HCl + HOCl
HOCl HCl + [O]
This [O] is called as nascent oxygen. This nascent oxygen is responsible for bleaching action.
NOTE: Since chlorine has the ability to kill harmful micro-organism it
...more
Similar Questions for you
HClO4 is the most acidic compound.
Group number = 11 (Atomic number = 111)
Heavier element of p block do not from pπ− pπ bonds as their atomic orbital are so large and diffius that they cannot have effecitve overlapping.
The inertness of
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers