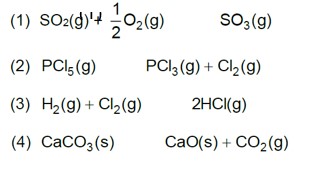
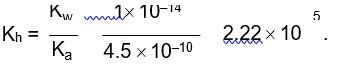7.31. Dihydrogen gas used in Haber's process is produced by reacting methane from natural gas with high temperature steam. The first stage of two stage reaction involves the formation of CO and H2 In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction.
CO (g) + H2O(g) ⇌ CO2(g) + H2(g)
If a reaction vessel at 400°C is charged with an equimolar mixture of CO and steam so that PCO = PH2O = 4.0 bar, what will be the partial pressure of H2 at equilibrium? Kp = 10.1 at 400°C.
7.31. Dihydrogen gas used in Haber's process is produced by reacting methane from natural gas with high temperature steam. The first stage of two stage reaction involves the formation of CO and H2 In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction.
CO (g) + H2O(g) ⇌ CO2(g) + H2(g)
If a reaction vessel at 400°C is charged with an equimolar mixture of CO and steam so that PCO = PH2O = 4.0 bar, what will be the partial pressure of H2 at equilibrium? Kp = 10.1 at 400°C.
Let the partial pressures of CO and H2 be p.
CO (g) + H2?O(g) ? CO2?(g) + H2?(g)
Initial conc. 4 bar 4 bar 0 0
At equilibrium 4-p 4-p p p
The expression for the equilibrium constant is
Kp? = ?PCO2 ??PH2??? / PCO?PH2?O
Similar Questions for you
0.01 M NaOH,
M = 1 * 10-2
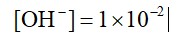
pOH = 2
pH = 2
Kp = Kc (RT)Dng
36 * 10–2 = Kc (0.0821 * 300)–1
Kc = 0.36 * 0.0821 * 300 = 8.86 » 9
A(g) ->B(g) + (g)
Initial moles n 0 &nbs
On increasing pressure, equilibrium moves in that direction where number of gaseous moles decreases.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering