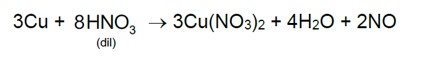7.33 Balance the following equation:
7.33 Balance the following equation:
-
1 Answer
-
7.33
XeF6 + H2O XeO2F2 + HF
Balanced equation: XeF6 + 2H2O XeO2F2 +4HF. The steps for balancing the reaction are as follows:
1. The main element is First, check if the atoms of Xe on both sides are balanced. YES, they are.
2. Then take another element i.e. F. It is not balanced on the right side, there are 3 atoms of F missing. So, to balance it multiply HF by 4.
3. Now check the other secondary atoms which are oxygen and hydrogen. Oxygen is not balanced on the left side as there are 2 O atoms so multiply H2O by
4. Now check for the hydrogen They are balanced on both sides.
Similar Questions for you
HClO4 is the most acidic compound.
Group number = 11 (Atomic number = 111)
Heavier element of p block do not from pπ− pπ bonds as their atomic orbital are so large and diffius that they cannot have effecitve overlapping.
The inertness of
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers