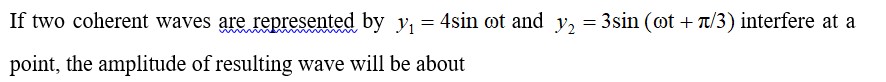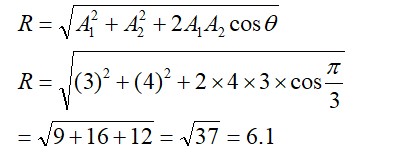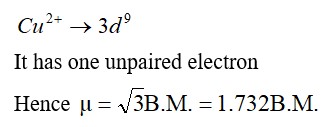8.9 Explain why Cu+ ion is not stable in aqueous solutions?
8.9 Explain why Cu+ ion is not stable in aqueous solutions?
8.9 The stability in aqueous condition depends on the hydration energy of the ions when they bond to the water molecules. And, the hydration energy is the amount of heat released as an ionic substance is dissolved and its constituent ions are hydrated or surrounded by water molecules.
Now, in Cu2+ an
Similar Questions for you
K2Cr2O7 + H2O2 + H2SO4->
Potassium permanganate in alkaline medium oxidise lodide to lodate.
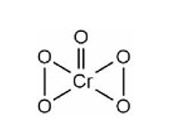
Compound A is
KMnO4 decomposes upon heating at 513 K and forms K2MnO4 and MnO2.
2KMnO4
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering