Assertion (A): If aluminium atoms replace a few silicon atoms in three dimensional network of silicon dioxide, the overall structure acquires a negative charge.
Reason (R): Aluminium is trivalent while silicon is tetravalent.
(A) Both A and R are true and R is the correct explanation of A.
(B) Both A and R are true but R is not the correct explanation of A.
(C) Both A and R are not correct.
(D) A is not correct but R is correct.
Assertion (A): If aluminium atoms replace a few silicon atoms in three dimensional network of silicon dioxide, the overall structure acquires a negative charge.
Reason (R): Aluminium is trivalent while silicon is tetravalent.
(A) Both A and R are true and R is the correct explanation of A.
(B) Both A and R are true but R is not the correct explanation of A.
(C) Both A and R are not correct.
(D) A is not correct but R is correct.
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
(A)
In aluminium silicate, silicon is doped with group 13 elements and as aluminium is trivalent and silicon is tetravalent so, on replacing aluminium by Silicon, a negatively charged structure will be obtained. Both the st
Similar Questions for you
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
(B)
Silicones are a class of silicon polymers that contain the repeating unit (-R 2 SiO-). Silicones are water repellent in nature because they are surrounded by non-polar alkyl groups, so A and R are both correct, but R is
This is a Matching Type Questions as classified in NCERT Exemplar
(i)→ (b), (ii) → (c), (iii) → (b), (iv) → (a) (v)→ (b) (vi)→ (c)
This is a Matching Type Questions as classified in NCERT Exemplar
(i)→ (c) (ii)→ (d) (iii)→ (a) (iv)→ (e) (v)→ (b)
This is a Matching Type Questions as classified in NCERT Exemplar
(i)- (e), (ii)- (c), (iii)- (d), (iv)- (a) & (b)
This is a Multiple Choice Questions as classified in NCERT Exemplar
(B) & (D)
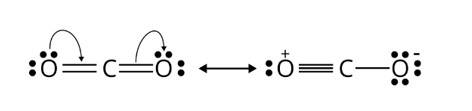
The following is the resonance structure of CO2.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering
