Match the species given in Column I with the properties mentioned in Column II.
Column I
Column II
(i) BF4−
(a) Oxidation state of central atom is +4
(ii) AlCl3
(b) Strong oxidising agent
(iii) SnO
(c) Lewis acid
(iv) PbO2
(d) Can be further oxidised
(e) Tetrahedral shape
Match the species given in Column I with the properties mentioned in Column II.
|
Column I |
Column II |
|
(i) BF4− |
(a) Oxidation state of central atom is +4 |
|
(ii) AlCl3 |
(b) Strong oxidising agent |
|
(iii) SnO |
(c) Lewis acid |
|
(iv) PbO2 |
(d) Can be further oxidised |
|
(e) Tetrahedral shape |
This is a Matching Type Questions as classified in NCERT Exemplar
(i)- (e), (ii)- (c), (iii)- (d), (iv)- (a) & (b)
Similar Questions for you
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
(B)
Silicones are a class of silicon polymers that contain the repeating unit (-R 2 SiO-). Silicones are water repellent in nature because they are surrounded by non-polar alkyl groups, so A and R are both correct, but R is
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
(A)
In aluminium silicate, silicon is doped with group 13 elements and as aluminium is trivalent and silicon is tetravalent so, on replacing aluminium by Silicon, a negatively charged structure will be obtained. Both the st
This is a Matching Type Questions as classified in NCERT Exemplar
(i)→ (b), (ii) → (c), (iii) → (b), (iv) → (a) (v)→ (b) (vi)→ (c)
This is a Matching Type Questions as classified in NCERT Exemplar
(i)→ (c) (ii)→ (d) (iii)→ (a) (iv)→ (e) (v)→ (b)
This is a Multiple Choice Questions as classified in NCERT Exemplar
(B) & (D)
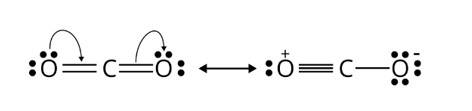
The following is the resonance structure of CO2.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering
