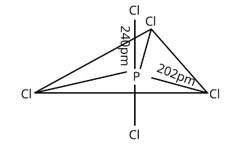
In PCl5 , phosphorus is in sp3d hybridised state but all its five bonds are not equivalent. Justify your answer with reason.
In PCl5 , phosphorus is in sp3d hybridised state but all its five bonds are not equivalent. Justify your answer with reason.
-
1 Answer
-
This is a short answer type question as classified in NCERT Exemplar
Three P—Cl bonds are arranged in one plane at a 1200 angle. These bonds are known as equatorial bonds because they are formed between two points. The remaining two P—Cl bonds, one above the equatorial plane and the other below it, form 90° angle with the plane. Axial bonds are the name for these types of bonds. Because axial bond pairs are subjected to greater repulsive interaction than equatorial bond pairs, axial bonds are slightly longer and hence slightly weaker than equatorial bonds, making the PCl5 molecule more
...more
Similar Questions for you
HClO4 is the most acidic compound.
Group number = 11 (Atomic number = 111)
Heavier element of p block do not from pπ− pπ bonds as their atomic orbital are so large and diffius that they cannot have effecitve overlapping.
The inertness of
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers