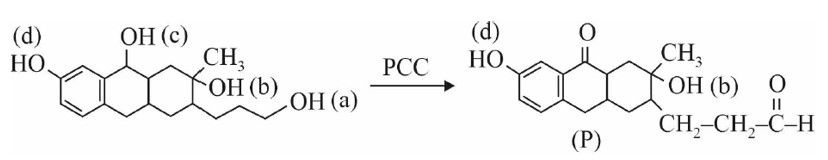
Match the following rules with their statements :
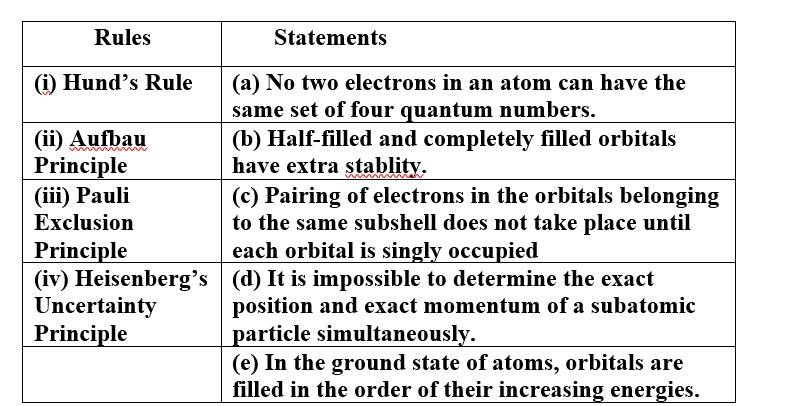
Match the following rules with their statements :

-
1 Answer
-
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Hund's Rule (c) Pairing of electrons in the orbitals belonging to the same subshell does not take place until each
orbital is singly occupied.
(ii) Aufbau Principle (e) In the ground state of atoms, orbitals are filled in the order of their increasing energies.
(iii)
...more
Similar Questions for you
Kindly go through the answers
(7.00)
Kindly consider the following Image
In 4d orbital, n = 4 and
Radial nodes =
Radial nodes = 4 – 2 – 1 = 1
And angular nodes,
Here, number of unpaired electrons, n = 1
Spin only moment ;
= 173 * 10-2 B.M
=
= (At constant pressure)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers