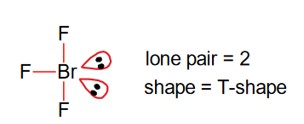
The hybridisations of the atomic orbitals of nitrogen in
respectively are:
The hybridisations of the atomic orbitals of nitrogen in respectively are:
Option 1 -
sp3, sp and sp2
Option 2 -
sp, sp2 and sp3
Option 3 -
sp2, sp and sp3
Option 4 -
sp3, sp2 and sp
Similar Questions for you
Hybridization of P in PF5 is sp3d, so value of y = 1
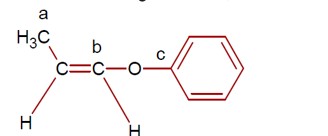
Hybridisation of carbon a, b, and c respectively are sp³, sp² and sp².
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers