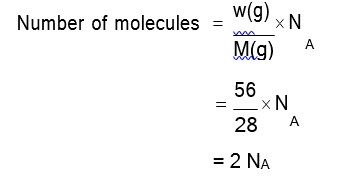The number of N atoms in 681 g of C7H5N3O6 is x * 1021. The value of x is___________.
(NA = 6.02 * 1023 mol1) (Nearest Integer)
The number of N atoms in 681 g of C7H5N3O6 is x * 1021. The value of x is___________.
(NA = 6.02 * 1023 mol1) (Nearest Integer)
3 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
R
Answered by
5 months ago
Molar mass of C7H5N3O6 = 12 * 7 + 1 * 5 + 14 * 3 + 16 * 6 = 84 + 42 + 96 = 227 g/mol
Number of moles = = 3 mol
Similar Questions for you
x + y + 3z = xyz3
1 mole 1 mole 0.05 mole
here z is limiting reagent.
mole z gives 1 mole xyz3
mass of xyz3 = n × molecular mass
=
= 0
-SO? H acts as a cation exchanger.
-NH? acts as an anion exchanger.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Some Basic Concepts of Chemistry 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering