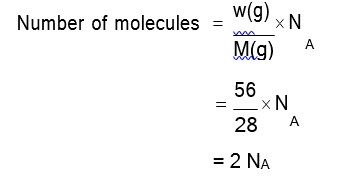The reaction 2A + B₂ → 2AB is an elementary reaction.
For a certain quantity of reactants, if the volume of the reaction vessel is reduced by a factor of 3, the rate of reaction increases by a factor of ______.
The reaction 2A + B₂ → 2AB is an elementary reaction.
For a certain quantity of reactants, if the volume of the reaction vessel is reduced by a factor of 3, the rate of reaction increases by a factor of ______.
-SO? H acts as a cation exchanger.
-NH? acts as an anion exchanger.
Similar Questions for you
x + y + 3z = xyz3
1 mole 1 mole 0.05 mole
here z is limiting reagent.
mole z gives 1 mole xyz3
mass of xyz3 = n × molecular mass
=
= 0
Molar mass of C7H5N3O6 = 12 × 7 + 1 × 5 + 14 × 3 + 16 × 6 = 84 + 42 + 96 = 227 g/mol
Number of moles = = 3 mol
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Some Basic Concepts of Chemistry 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering