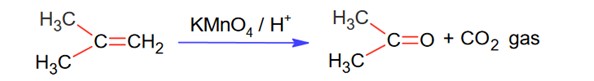Two isomers 'A' and 'B' with molecular formula C4H8 give different products on oxidation with KMnO4 is acidic medium. Isomer 'A' on reaction with KMnO4/H+ results in effervescence of a gas and given ketone. The compound 'A' is
Two isomers 'A' and 'B' with molecular formula C4H8 give different products on oxidation with KMnO4 is acidic medium. Isomer 'A' on reaction with KMnO4/H+ results in effervescence of a gas and given ketone. The compound 'A' is
Option 1 - <p>But-1-ene</p>
Option 2 - <p>Cis-But-2-ene</p>
Option 3 - <p>Trans-But-2-ene</p>
Option 4 - <p>2-methyl propene.</p>
1 Views|Posted 6 months ago
Asked by Shiksha User
1 Answer
A
Answered by
6 months ago
Correct Option - 4
Detailed Solution:
C4H8 (DOU = 1 so 1 double bond exist since it gives KMnO4 test)
Similar Questions for you
Reaction is
Mass of product, 1, 2, 2- tetrobromopropane obtained
=
= 3.30375
= 3.0375 × 10-1
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering