What are the major differences between valence bond theory & Molecular Orbital Theory?
What are the major differences between valence bond theory & Molecular Orbital Theory?
-
1 Answer
-
You can check the below given table for the comarative differences between valence bond theory and molecular orbital theory.
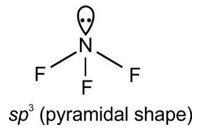
Feature Valence Bond Theory (VBT) Molecular Orbital Theory (MOT) Basic Concept Overlap of atomic orbitals. Atomic orbitals combine to form molecular orbitals Bond Formation Due to head-on (? ) or sideways (? ) overlap of atomic orbitals. Linear combination of atomic orbitals (LCAO) to form bonding and antibonding MOs. Explanation of Magnetic Behavior Often fails to explain magnetism of molecules (e.g., O? is paramagnetic). Accurately explains paramagnetism/diamagnetism (O? is paramagnetic due to unpaired electrons in antibonding orbitals). Bond Order Not clearly defined. Bond order = ½ (No. of bonding electrons - No. of antibonding electrons) Electron Delocalization Electrons are localized between two atoms. Electrons may be delocalized over multiple atoms. Energy Consideration Considers only overlapping orbitals and their energy. Considers combination and energy differences of atomic orbitals. Applicability Works well for simple molecules like H? , HF, etc. Better for explaining molecules like O? , N? , and ions like NO? , CN?
Similar Questions for you
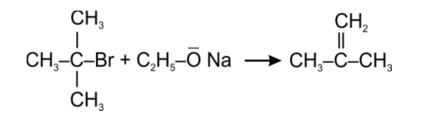
Tertiary haloalkane does not undergo SN2 reaction
He2 has zero bond order hence it does not exist.
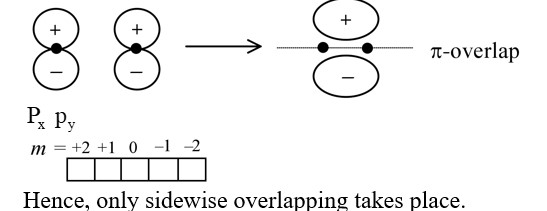
For overlap, the lobes of the atomic orbitals are perpendicular to the line joining the nuclei.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers