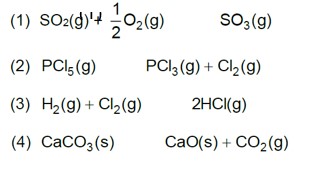
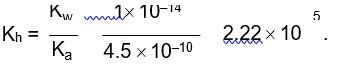Which of the following is not a general characteristic of equilibria involving physical processes?
(i) Equilibrium is possible only in a closed system at a given temperature.
(ii) All measurable properties of the system remain constant.
(iii) All the physical processes stop at equilibrium.
(iv) The opposing processes occur at the same rate and there is dynamic but stable condition.
Which of the following is not a general characteristic of equilibria involving physical processes?
(i) Equilibrium is possible only in a closed system at a given temperature.
(ii) All measurable properties of the system remain constant.
(iii) All the physical processes stop at equilibrium.
(iv) The opposing processes occur at the same rate and there is dynamic but stable condition.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iii)
A physical equilibrium is having dynamic nature, both forward and reverse process occur at equal rates. Some kind of motion is always present. The individual molecules continuously move from one phase to another.
Similar Questions for you
0.01 M NaOH,
M = 1 * 10-2
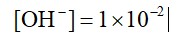
pOH = 2
pH = 2
Kp = Kc (RT)Dng
36 * 10–2 = Kc (0.0821 * 300)–1
Kc = 0.36 * 0.0821 * 300 = 8.86 » 9
A(g) ->B(g) + (g)
Initial moles n 0 &nbs
On increasing pressure, equilibrium moves in that direction where number of gaseous moles decreases.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Seven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering