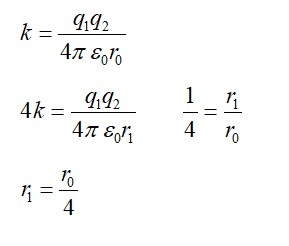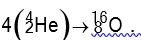13.28 Consider the D–T reaction (deuterium–tritium fusion)
(a) Calculate the energy released in MeV in this reaction from the data:
m( =2.014102 u
m( =3.016049 u
(b) Consider the radius of both deuterium and tritium to be approximately 2.0 fm. What is the kinetic energy needed to overcome the coulomb repulsion between the two nuclei? To what temperature must the gas be heated to initiate the reaction?
(Hint: Kinetic energy required for one fusion event =average thermal kinetic energy available with the interacting particles = 2(3kT/2); k = Boltzmann's constant, T = absolute temperature.)
13.28 Consider the D–T reaction (deuterium–tritium fusion)
(a) Calculate the energy released in MeV in this reaction from the data:
m( =2.014102 u
m( =3.016049 u
(b) Consider the radius of both deuterium and tritium to be approximately 2.0 fm. What is the kinetic energy needed to overcome the coulomb repulsion between the two nuclei? To what temperature must the gas be heated to initiate the reaction?
(Hint: Kinetic energy required for one fusion event =average thermal kinetic energy available with the interacting particles = 2(3kT/2); k = Boltzmann's constant, T = absolute temperature.)
13.28 The equation for deuterium-tritium fusion is given as:
It is given that
Mass of ( = 2.014102 u
Mass of ( , 3.016049 u
Mass of ( = 4.002603 u
Mass of ( = 1.008665 u
Q-value of the given D-T reaction is:
Q =
=
= 0.018883
= 17.59 MeV
Radius of the deuterium and tritium, r = 2 m
Distance
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering