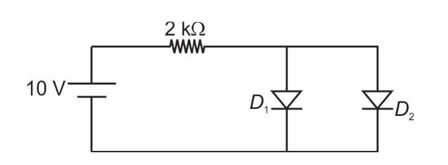
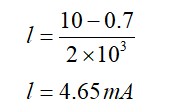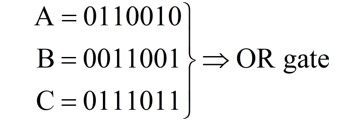14.8 The number of silicon atoms per m3 is 5 * 1028. This is doped simultaneously with 5 * 1022 atoms per m3 of Arsenic and 5 * 1020 per m3 atoms of Indium. Calculate the number of electrons and holes. Given that ni = 1.5 * 1016 m–3. Is the material n type or p type?
14.8 The number of silicon atoms per m3 is 5 * 1028. This is doped simultaneously with 5 * 1022 atoms per m3 of Arsenic and 5 * 1020 per m3 atoms of Indium. Calculate the number of electrons and holes. Given that ni = 1.5 * 1016 m–3. Is the material n type or p type?
14.8 Number of silicone atoms, = 5 /
Number of arsenic atoms, = 5 /
Number of indium atoms, = 5 /
Number of thermally generated atoms, = 1.5 /
Hence number of electrons, = 5 1.5 = 4.99
Number of holes =
In thermal equilibrium, the concentration of electrons and holes in a semiconductor ar
Similar Questions for you
A n-p-n transistor can be used as amplifier.
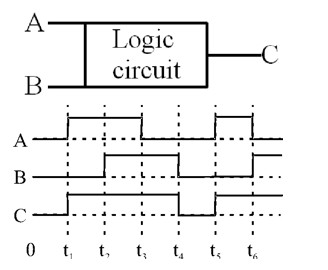
Corresponding binary numbers of given waveforms.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2026
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering