7.2 In the HCl molecule, the separation between the nuclei of the two atoms is about 1.27 Å (1 Å = 10-10 m). Find the approximate location of the CM of the molecule, given that a chlorine atom is about 35.5 times as massive as a hydrogen atom and nearly all the mass of an atom is concentrated in its nucleus.
7.2 In the HCl molecule, the separation between the nuclei of the two atoms is about 1.27 Å (1 Å = 10-10 m). Find the approximate location of the CM of the molecule, given that a chlorine atom is about 35.5 times as massive as a hydrogen atom and nearly all the mass of an atom is concentrated in its nucleus.
14 Views|Posted 9 months ago
Asked by Shiksha User
1 Answer
V
Answered by
9 months ago
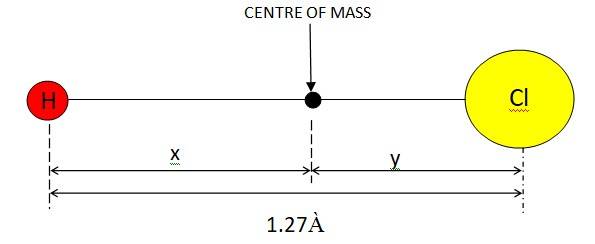
Let us assume that H atom and Cl atom are at a distance of x and y respectively from the CM (Centre of Mass).
If mass of the H atom = m, mass of the Cl atom = 35.5m
Given x + y = 1,27 À
Let us assume that the centre of mass of the given molecule lies at the origin. Therefore,
We can have, : (my+35.5mx)
Similar Questions for you
Use formula for M.I.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

physics ncert solutions class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering