A hot air balloon is a sphere of radius 8 m. The air inside is at a temperature of 60°C. How large a mass can the balloon lift when the outside temperature is 20°C? (Assume air is an ideal gas, R = 8.314 J mole–1K-1, 1 atm. = 1.013*105 Pa; the membrane tension is 5 N m-1.)
A hot air balloon is a sphere of radius 8 m. The air inside is at a temperature of 60°C. How large a mass can the balloon lift when the outside temperature is 20°C? (Assume air is an ideal gas, R = 8.314 J mole–1K-1, 1 atm. = 1.013*105 Pa; the membrane tension is 5 N m-1.)
This is a long answer type question as classified in NCERT Exemplar
Let the pressure inside the ballon be P1 and the outside pressure be Po, then excess pressure is Pi-Po =2S/r
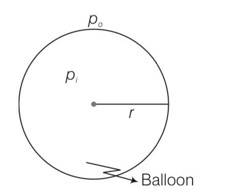
Considering the air to be an ideal gas piV = niRTi = where, V is the volume of the air inside the balloon, ni is the number o
Similar Questions for you
Surface tension is the force acting on the surface of the liquid.
Bernoulli's principle states that in a steady flow, the sum of pressure, kinetic energy per unit volume, and potential energy per unit volume remains constant.
Yes, the Mechanical properties of fluids class 11th physics is important in NEET. On average, 1-2 questions would be asked from this chapter, which you can cover from the Class 11th Mechanical Properties of Fluids notes.
The main mechanical properties of fluids are exerting pressure, resisting flow or viscosity, forming surface tension, following Bernoulli's principle, and moving in a streamline.
Since velocity does not change, so acceleration will be zero.
mg = FB + Fv
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics NCERT Exemplar Solutions Class 11th Chapter Ten 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering
