A monoatomic gas at pressure P and volume V is suddenly compressed to one eight of its original volume. The final pressure at constant entropy will be:
A monoatomic gas at pressure P and volume V is suddenly compressed to one eight of its original volume. The final pressure at constant entropy will be:
Constant Entropy means Adiabatic process
P2 = 32 P
Similar Questions for you
Process-AB Isobaric, &n
from newtan’s law of cooling :
Using average value :
Efficiency
-(i)
After the change :- -(ii)
--(ii)
At Resonance
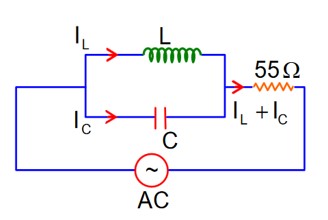
XL = XC
then lL = lC
Now phasor diagram
for L & C
So, Net current = zero
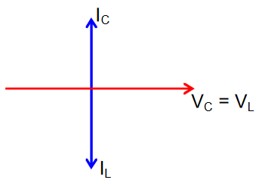
Therefore current through R circuit at resonance will be zero
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics NCERT Exemplar Solutions Class 12th Chapter Twelve 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering