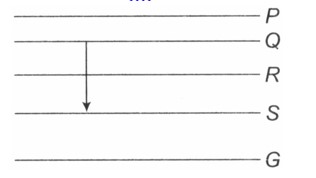
Figure shows the energy levels P,Q,R,S and G of an atom where G is the ground state. A red line in the emission spectrum of the atom can be obtained by an energy level change from Q to S. A blue line can be obtained by following energy level change
Figure shows the energy levels P,Q,R,S and G of an atom where G is the ground state. A red line in the emission spectrum of the atom can be obtained by an energy level change from Q to S. A blue line can be obtained by following energy level change
Option 1 - <p>P to Q</p>
Option 2 - <p>Q to R</p>
Option 3 - <p>R to S</p>
Option 4 - <p>R to G</p>
2 Views|Posted 4 months ago
Asked by Shiksha User
No answers yet.
Can you answer this question?Similar Questions for you
Density of nucleus is constant.
r = 0.5Å = 0.5×10? ¹? m
v = 2.2×10? m/s
I =?
t = 2πr/v
I = e/t = ev/2πr = (1.6×10? ¹? × 2.2×10? )/ (2 × 22/7 × 0.5×10? ¹? )
I ≈ 1.12×10? ³ A = 1.12 mA = 112 × 10? ² mA
(1) n1 = 3, n2 = 2
(2) n1 = 4, n2 = 3
(3) n1 = 2, n2 = 1
(4) n1 = 5, n2 = 4
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering