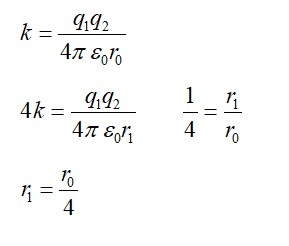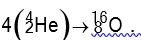How does the nuclear force contribute to nuclear stability?
How does the nuclear force contribute to nuclear stability?
Asked by Shiksha User
-
1 Answer
-
Nuclear force is important to maintain the stability of an atomic nucleus for the following reasons:
- A nucleus has positively charged sub-particles called protons. Since like charges repel each other, a nucleus, in ideal scenario, should fall apart because protons won't be able to coexist in the nucleus. However, nuclear force is extremely strong at very short range. Due to this reason, protons stay bound within nucleus and therefore, nucleus remains stable.
- Nuclear force is responsible for binding energy that holds the nucleons together within a nucleus. Higher binding energy will result in a more stable nucleus.
- The nuclear force shows
...more
Similar Questions for you
A
alok kumar singh
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
A
alok kumar singh
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers