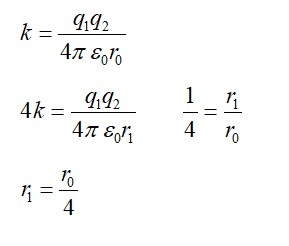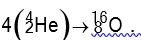How does the number of neutrons affect the stability of a nucleus?
How does the number of neutrons affect the stability of a nucleus?
-
1 Answer
-
The number of neutrons in nucleus has an important role in determining the stability of nucleus. Stability of nucleus is affected by the balance between number of protons and neutrons and the number of nucleons. In lighter elements, the most stable nuclei has roughly equal number of protons and neutrons. For instance, Carbon-12 is the most common isotope of carbon with 6 protons and 6 neutrons.
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers