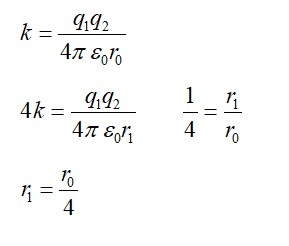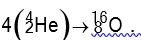Sometimes a radioactive nucleus decays into a nucleus which itself is radioactive. An example is
38sulphur 38Cl 38Ar(stable)
Assume that we start 1000 38S nuclei at time t=0. The number of 38Cl is of count zero at t=0 and will be again zero at t= at what value of t, would the number of counts be a a maximum?
Sometimes a radioactive nucleus decays into a nucleus which itself is radioactive. An example is
38sulphur 38Cl 38Ar(stable)
Assume that we start 1000 38S nuclei at time t=0. The number of 38Cl is of count zero at t=0 and will be again zero at t= at what value of t, would the number of counts be a a maximum?
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- let 38S have N1 active nuclei and 38Cl have N2 active nuclei
+
N1=N0
+
Multiplying by and then integrating both sides we got
N2=
After solving it we get time t= (log )/
t= =
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering