The change in the magnitude of the volume of an ideal gas when a small additional pressure is applied at a constant temperature, is the same as the change when the temperature is reduced by a small quantity at constant pressure. The initial temperature and pressure of the gas were and 2 atm. respectively. If then value of in (K/atm) is ....
The change in the magnitude of the volume of an ideal gas when a small additional pressure is applied at a constant temperature, is the same as the change when the temperature is reduced by a small quantity at constant pressure. The initial temperature and pressure of the gas were and 2 atm. respectively. If then value of in (K/atm) is ....
Similar Questions for you
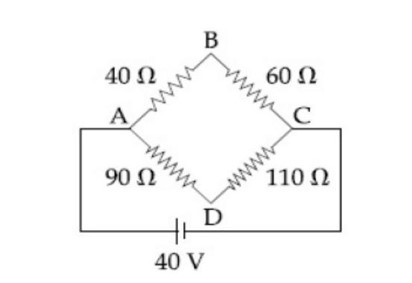
Process-AB Isobaric, &n
from newtan’s law of cooling :
Using average value :
Efficiency
-(i)
After the change :- -(ii)
--(ii)
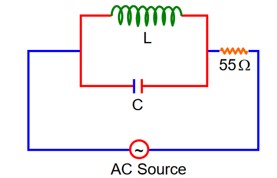
At Resonance
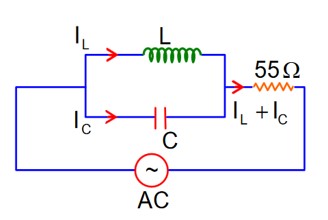
XL = XC
then lL = lC
Now phasor diagram
for L & C
So, Net current = zero
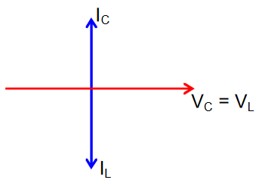
Therefore current through R circuit at resonance will be zero
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics NCERT Exemplar Solutions Class 12th Chapter Six 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering