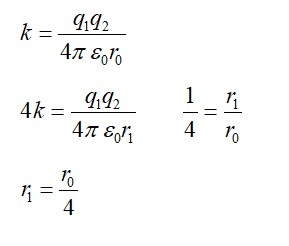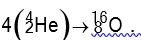The deuteron is bound by nuclear forces just as H-atom is made up of p and e bound by electrostatic forces. If we consider the force between neutron and proton in a deuteron as given in the form a coulomb potential but with an effective charge e'.
F= ke'2/r estimate the value of e'/e given that the the binding energy of a duetron is 2.2MeV.
The deuteron is bound by nuclear forces just as H-atom is made up of p and e bound by electrostatic forces. If we consider the force between neutron and proton in a deuteron as given in the form a coulomb potential but with an effective charge e'.
F= ke'2/r estimate the value of e'/e given that the the binding energy of a duetron is 2.2MeV.
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation-E=
If proton and neutron had charge e each and were governed by the same electrostatic force, then in the above equation we would need relace m
So m' = =M/2=918m
Hence binding energy= 918me'4/8
Dividing eqn
918 ( 4= =
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering