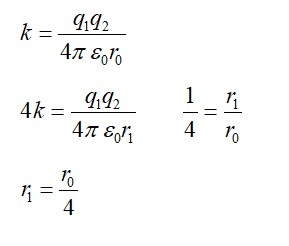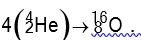Tritium is an isotope of hydrogen whose nucleus triton contains 2 neutrons and 1 proton. Free neutrons decay into p + e + n . If one of the neutrons in Triton decays, it would transform into He3 nucleus. This does not happen. This is because
(a) Triton energy is less than that of a He3 nucleus
(b) The electron created in the beta decay process cannot remain in the nucleus
(c) Both the neutrons in triton have to decay simultaneously resulting in a nucleus with 3 protons, which is not a He3 nucleus.
(d) Free neutrons decay due to external perturbations which is absent in triton nucleus
Tritium is an isotope of hydrogen whose nucleus triton contains 2 neutrons and 1 proton. Free neutrons decay into p + e + n . If one of the neutrons in Triton decays, it would transform into He3 nucleus. This does not happen. This is because
(a) Triton energy is less than that of a He3 nucleus
(b) The electron created in the beta decay process cannot remain in the nucleus
(c) Both the neutrons in triton have to decay simultaneously resulting in a nucleus with 3 protons, which is not a He3 nucleus.
(d) Free neutrons decay due to external perturbations which is absent in triton nucleus
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (a)
Explanation – tritium contain 1 proton and 2 neutron but when it disintegrates and remove 1 beta particle it will now contain 2 proton and 1 neutron so it will change into 2He3 but its energy will be less from it.
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering