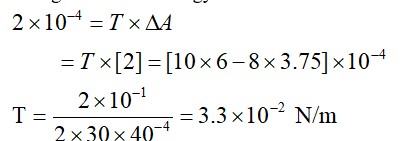Why couldn’t Rutherford explain the stability of an atom?
Why couldn’t Rutherford explain the stability of an atom?
-
1 Answer
-
Based on what classical electromagnetic theory says, we know that an accelerated charged particle must radiate energy or electromagnetic waves. That remains continuous.
By looking at the Rutherford’s atomic model we can assume that electrons would revolve around the nucleus, which in this logic, held in orbit by electrostatic attraction. The reason is that, their path is circular that’s always accelerating. That would mean the electron would start to lose energy and finally let the atom to implode. But that doesn’t happen. Atoms are stable and that’s where classical physics that led to Rutherford model fail
...more
Similar Questions for you
In Rutherford's model, electrostatic force provides the centripetal force:
∈
Kinetic Energy (K):
Potential Energy (U):
Total Energy (E):
Note that, E is the Epsilon symbol.
In the gold foil scattering experiment by Rutherford and his team, almost all alpha particles passed straight through the foil. There were minor deflections mostly, and only a small amount of them showed deflections at really large angles. Only a few of them rebounded. This was the main observation that led Rutherford to conclude that the positive charge and mass of the atom concentrate at a very tiny section of the atom. That’s the nucleus. The other observation was that the entire atom must be space where the alpha particles could go undeflected.
Kindly go through the solution
Change in surface energy = work done
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers