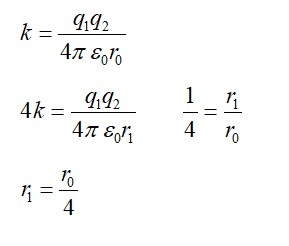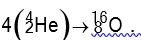Why do some elements have fractional atomic masses?
Why do some elements have fractional atomic masses?
-
1 Answer
-
Most elements exist as a mixture of isotopes in nature. Isotopes are atoms of same elements that have same number of protons but they have different numbers of neutrons. Since the number of neutrons may vary, different isotopes of same element have different atomic masses.
The atomic mass of an element is a weighted average of atomic masses of its isotopes considering their natural abundances. The weighted average is calculated by multiplying atomic mass of every isotope by its natural abundance and then adding these products.
The atomic mass is an average that accounts for different masses and abundances of isotopes that results in a fr
...more
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers