Ask & Answer: India's Largest Education Community
All Questions
New Question
5 months agoContributor-Level 7
The GATE Energy Science syllabus can be checked below -
- Energy resources and conversion technologies - fossil energy resources, nuclear energy resources, biomass, hydropower, etc.
- Energy storage, economics, environment, and efficiency - energy storage systems, energy management, environmental impacts of energy use, etc.
The GATE paper code of Energy Science is GATE XE-I.
New Question
5 months agoContributor-Level 7
Students can download GATE syllabus, following process below-
- Go to official website
- Select desired GATE paper (applying for)
- Download syllabus and start preparing for exam.
New Question
5 months agoContributor-Level 7
No, there is no change in GATE 2026 syllabus. However, a new sectional paper has been added under Engineering Sciences, XE, which is Energy Science and GATE paper code is XE-I. Candidates can download GATE syllabus 2026 PDF from official website- gate2026.iitg.ac.in.
New Question
5 months agoContributor-Level 6
LPU NEST is conducted multiple times in a year. There is no requirement to appear for the previous phase of the exam to appear for the next phase. The application form will be available for each phase separately. The candidates can log in to the official website and submit the application form, and appear for the exam. The exam is conducted from January to August.
New Question
5 months agoContributor-Level 9
Let we take of solution
Mass of solute = Volume × Density
= 0.5
= 0.525 gram
Mass of solution = 1 kg. [considering very dilute solution]
Mass of solvent = 1000 – 0.525 = 999.475 gram
New Question
5 months agoContributor-Level 10
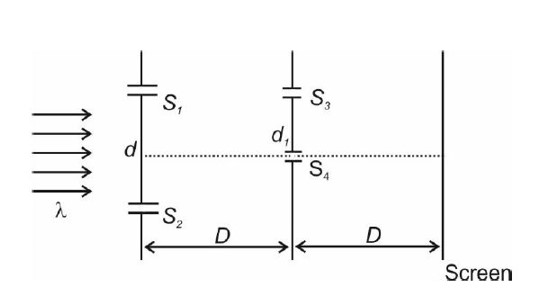
For maxima = y = (2n + 1)
For 1st maxima for l1 wavelength (n = 1)
- (1)
First maxima for l2 wavelength
- (2)
New Question
5 months agoNew Question
5 months agoContributor-Level 10
Let l = l0 cos wt
Then v = v0 sinwt
at t = 0, v = 0
but l = l0
a = 121 × 2
a = 242
New Question
5 months agoContributor-Level 9
Let a moles of SO2Cl2 is taken
Then no. of moles of H2SO4 = a moles
No. of moles of HCl = 2a moles
No. of moles of NaOH required = 2a + 2a = 4a = 16
New Question
5 months agoContributor-Level 10
Ib = 10 µA
IC = 1.5 mA
RL = 50 kW or (Rc)
Base – emitter voltage = 10 mv
=
Av = 750
New Question
5 months agoContributor-Level 9
B2H6 has 4 2 c -2e bonds and 2 3c-2e bonds.
Bridging (B-H) bonds have more value of bond- length then terminal (B – H) bonds
Bridging bonds are in one plane, but terminal bonds are in perpendicular plane.
Due to presence of (3c-2e) bonds, it behaves as electrons deficient and prone to get attached by lewis base.
New Question
5 months agoNew Question
5 months agoNew Question
5 months agoContributor-Level 10
Answer (3)
Particles having phase difference of will move with same speed.
New Question
5 months agoContributor-Level 9
Due to higher extent of polarization by Li+ and Mg2+, LiCl and Mgcl2 have covalent character. Therefore they are soluble in ethanol.
Due to very high value of lattice energy, LiF is having very less solubility in water.
New Question
5 months agoContributor-Level 9
The highest industrial consumption of hydrogen gas is in the synthesis of ammonia gas (Having manufacturing of N-based fertizers)
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts